20 Predict Which of the Following Has a Covalent Bond
Valence electrons are involved in both types of bonds. In covalent bonds atoms share electrons whereas is ionic bonds electrons are transferred between atoms.

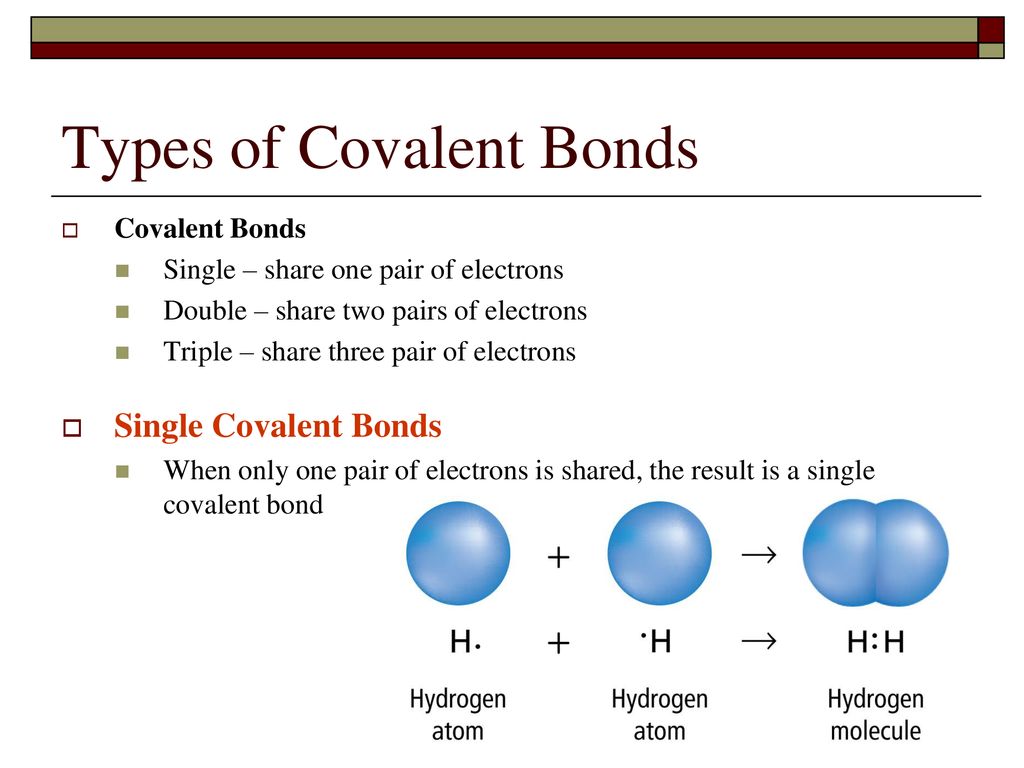
2 7 Single Double And Triple Covalent Bonds Covalent Bonding Chemistry Classroom Chemistry Lessons
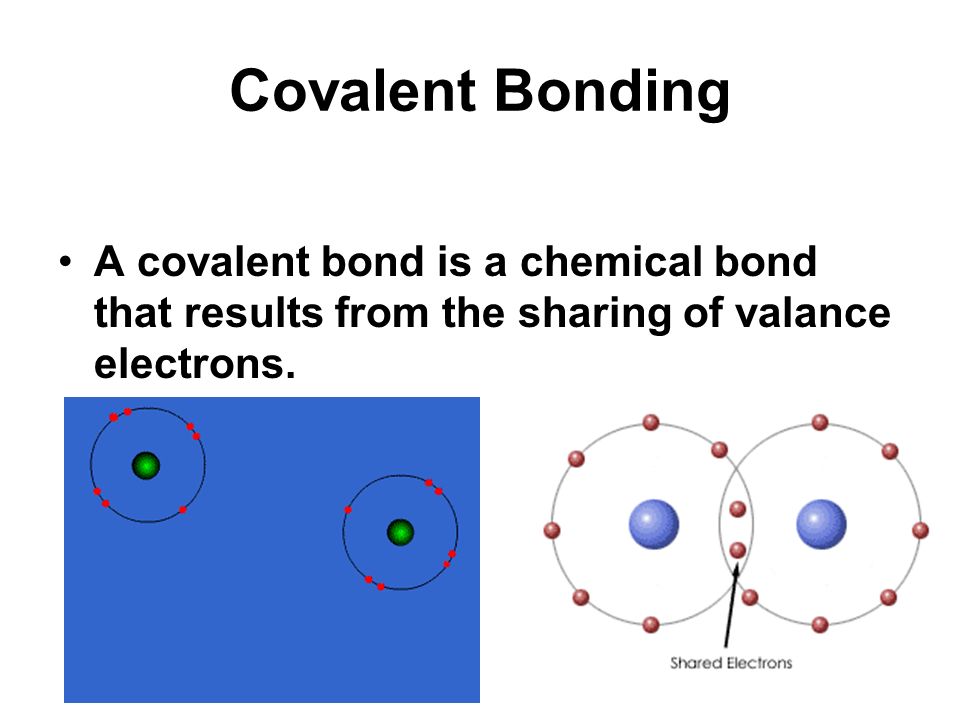
A chemical bond resulting from two atoms sharing one or more pairs of electrons.
. Magnesium electronegativity 131. Ionic Polar covalent Nonpolar covalent If you are not given electronegativity. SiCl 4 PCl 3 CaCl 2 CsCl CuCl 2 and CrCl 3.
A non metal can co. Up to 256 cash back a hydrogen bond. These electrons are simultaneously attracted by the two atomic nuclei.
D both atoms in the bond must be metals. B one atom in the bond must have higher electronegativity than the other atom. Starting on the far right we have two separate hydrogen atoms with a particular potential energy indicated by the red line.
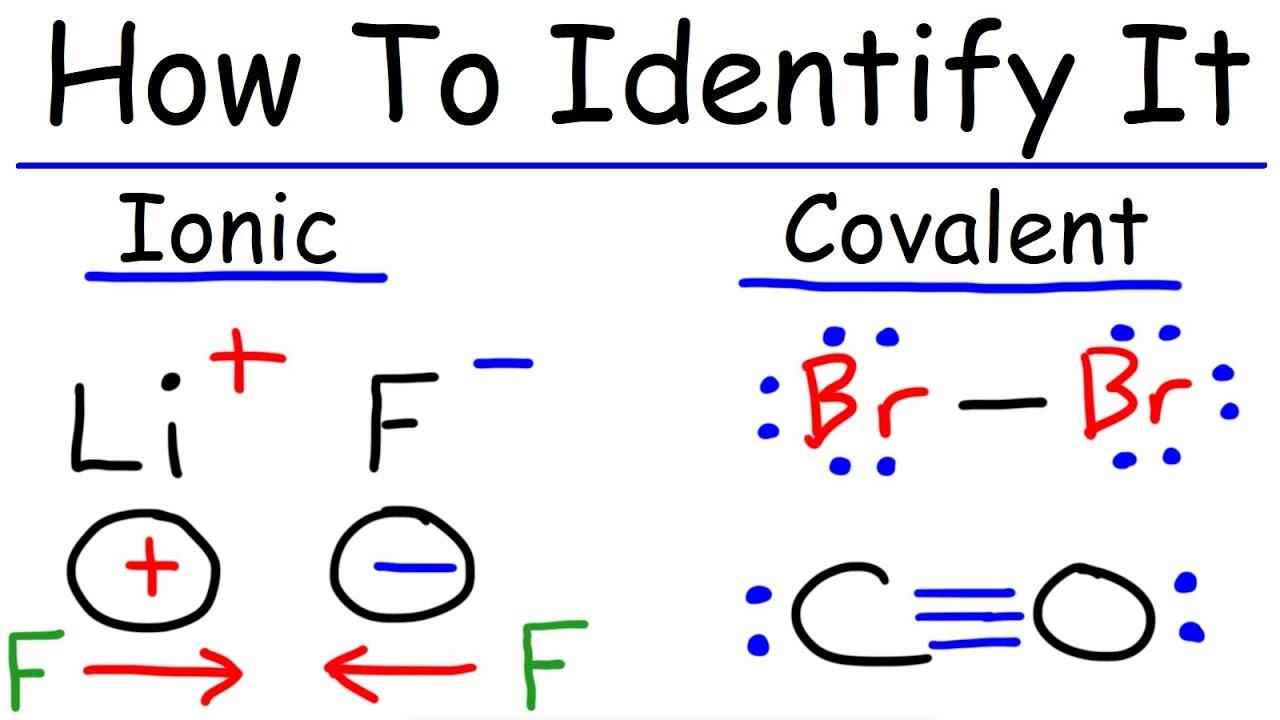
A chemical bond resulting from the electrostatic attraction of an anion and a cation. Polar covalent bonds can be said to have a charge separation or a bond dipole. KCl MgCl 2.
Example Exercise 121Bond Predictions. Two or more nonmetal atoms are attracted in covalent bonds. In a polar covalent bond A both atoms in the bond have the same level of electronegativity.
A Ammonia contains the nonmetals nitrogen and hydrogen. Chlorine electronegativity 316. Covalent Bonding 235 Chapter 8.
A sigma bond is a single covalent bond formed from the direct overlap. D all of the above. Other sets by this creator.
CHEM 1114 Chap 2. A Ionic bond C Polar covalent bond. The answer to your question is a Covalent b Ionic.
E none of the above 13 Predict which of the following has a covalent bond. Bond Polarity and Inductive EffectBond Polarity and Inductive Effect Nonpolar Covalent Bonds. D an enzyme is required to break this bond.
Kind of bond between C - C. Predict which of the following has a covalent bond refer to Periodic Table. 2 Predict which of the following has a covalent bond.
One way to predict whether a bond is ionic or covalent is to look how far apart the two atoms forming the bonds are in the periodic table. Carbon electronegativity 255. Difference in EN of atoms 2 Ionic Bonds.
N to N 30-3. If the difference is between 04 and 20 units the bond is classified as polar covalent and if the difference is more than 20 units the bond is substantially ionic. 2- Calculate the difference of electronegativities.
Predict which of the following has an covalent bond. The cation is deficient in electrons and the anion has extra electrons. A Cl Cl B Si Si C Ca Cl D Cr Br E P Cl.
Up to 256 cash back If the electronegativities differ by less than 04 units the bond can be classified as nonpolar covalent. Predict which of the following has an ionic bond. Draw an electron dot structure for each.
For example the hydrogen molecule H 2 contains a covalent bond between its two hydrogen atoms. Difference in EN 2 CH bonds relatively nonpolar C-O C-X bonds more electronegative elements are polarelectronegative elements are polar Bonding electrons shift toward. A polar covalent bond would form in which one of the following pairs of atoms.
NCl 3 ICl PCl 5 CCl 4 Using the periodic table predict whether the following chlorides are ionic or covalent. Covalent Bonding Solutions for Chapter 8 Questions for Review and Thought Review Questions 1. Up to 24 cash back covalent bonds.
B Magnesium nitride contains a metal Mg and a nonmetal N. Covalent bonds in which the bonding electrons are unequally shared are called polar covalent bonds. Explain how the VSEPR theory can be used to predict bond angles in the following covalently bonded molecules.
View the full answer. A metal ion and nonmetal ion are attracted in ionic bonds. We usually call those above 18 ionic but those very close to 18 or 19 still are polar covalent although more ionic than covalent.
All bonds lie along a continuum and have characteristics of both covalent and ionic. 10 Which of the following properties make this bond the ideal choice for linking amino acids together to form a polypeptide chain. A H 2 S B NH 3 C CH 4 D all of the above E none of the above.
D all of the above. There is another misconception. D all of the above.
Compare and contrast ionic bonds and cova-lent bonds. We classify chemical bonds as ionic polar covalent and nonpolar covalent based on the difference in electro-negativity between the bonded. Use the following table to look up the electronegativities of.
PVnRT TPVnR 20c 273 293 025000821 0020525 293 0333 87987 87987 x 0020525 1805 atm. C it is electrostatic. Up to 24 cash back The following molecules have single covalent bonds.
Between zero and 18 is olar covalent. C one atom must be a H atom. Predict which of the following has a covalent bond CaS CdS CoS.
Order the following covalent bonds from least to most polar. It follows that NH3has covalentbonds. Follow up Oh No.
Formation of Covalent Bonds. 14 Predict which of the following has a covalent bond. Bonds are not simply ionic or covalent.
D polar covalent bond. 1 single bond and 1 double bond 2 single bonds 1 single bond and 1 triple bond none of the above Predict which of the. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms.
H 2 O 2 b. 20 Predict which of the following has an covalent bond. An ionic bond and a covalent bond differ in the location of the electrons.
What type of chemical bond holds the atoms together within a water molecule. If delta EN electronegativity 18 or 19 that is about 50 ionic50 covalent. In fact there are NO 100 ionic bonds.
B nonpolar covalent bond. Illustrates why this bond is formed. A covalent bond consists of the mutual sharing of one or more pairs of electrons between two atoms.
Using the periodic table predict whether the following chlorides are ionic or covalent. We review their content and use your feedback to keep the quality high. If one atom is on the far left Group 1 or 2 and the other is on the far right Group 5 6 or 7 then the atoms will have large differences in EN and will form an ionic bond.
E none of the above. 1- Look for the electronegativities of these elements. B it is polar.
KCl NCl 3 ICl MgCl 2 PCl 5 and CCl 4. 20 A CH4 B NH3 C PH3 D ll of the above E none of the above. E it is reversible in water.
Contrast sigma bonds and pi bonds. D all of the above. Kind of bond between Mg -Cl.
Dec 3 2012. 12 Predict which of the following has a covalent bond. In ionic bonds there is no sharing of electrons.
A it is nonpolar. Steps to Determine Bond Type. Predict which of the following has an ionic bond.
Atoms with similar EN Polar Covalent Bonds. Look at the atoms in the molecule and look up their electronegativity. All bonds involve some degree of sharing of electrons although some bonds may be more polar than others.
The correct option is D all of the above. Take the difference of electronegativity between the two atoms. Such a bond dipole can be represented by an arrow with the arrowhead pointing towards the partially H Cl 22 32 H Cl.
A CoO B NO C ZnO D all of the above E none of the above. HCl KCl NaCl all of the above none of the above Draw the structural formula for the chloride ion ClO_2- and state the type of bonds in a.

Covalent Bond Definition Types And Examples

Covalent Bonding Covalent Bonding And Covalent Nomenclature Ppt Download
Ionic And Covalent Bonding Chemistry Youtube

Question Video Calculating The Number Of Covalent Bonds Possible From The Number Of Valence Electrons Nagwa

Ionic Bonds Polar Covalent Bonds And Nonpolar Covalent Bonds Youtube

Covalent Bond Definition Types And Examples

Covalent Bond Definition Properties Examples Facts Britannica

Covalent Bonding Ppt Video Online Download

Covalent Compounds Covalent Bond Properties Examples With Videos

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry

Covalent Bonding Covalent Bonding And Covalent Nomenclature Ppt Download

Crystal Types Of Bonds Britannica
Covalent Bonding Chemistry Chapter Ppt Download

Ionic Vs Covalent Bonds Ionic Vs Covalent Bonds Covalent Bonding Covalent Bonds

Covalent Bonding Ppt Video Online Download

Single Covalent Bond Molecule Examples What Is A Single Bond Video Lesson Transcript Study Com

Covalent Bond Ck 12 Foundation


Comments
Post a Comment